Biochemical and Genetic Basis of Antimicrobial Resistance
Throughout human history, infections have remained the primary cause of diseases. The discovery, introduction, and subsequent widespread use of antibiotics aimed at eliminating the problems of infections. However, several bacterial species of animal and human origin have been able to develop several mechanisms to be resistant to antimicrobial agents.
Many threatening pathogens are now resistant to multiple classes of antimicrobial agents. A study tested 500 Streptomyces strains isolated from soil against 21 antibiotics of different class found that all the strains were multidrug resistant to at least 7 of the tested antibiotics (D’Costa et al., 2006). These multidrug-resistant (MDR) organisms cause infections that may compromise effective therapy and limit treatment options.
Economic and Mortality Impacts of Antimicrobial Resistance
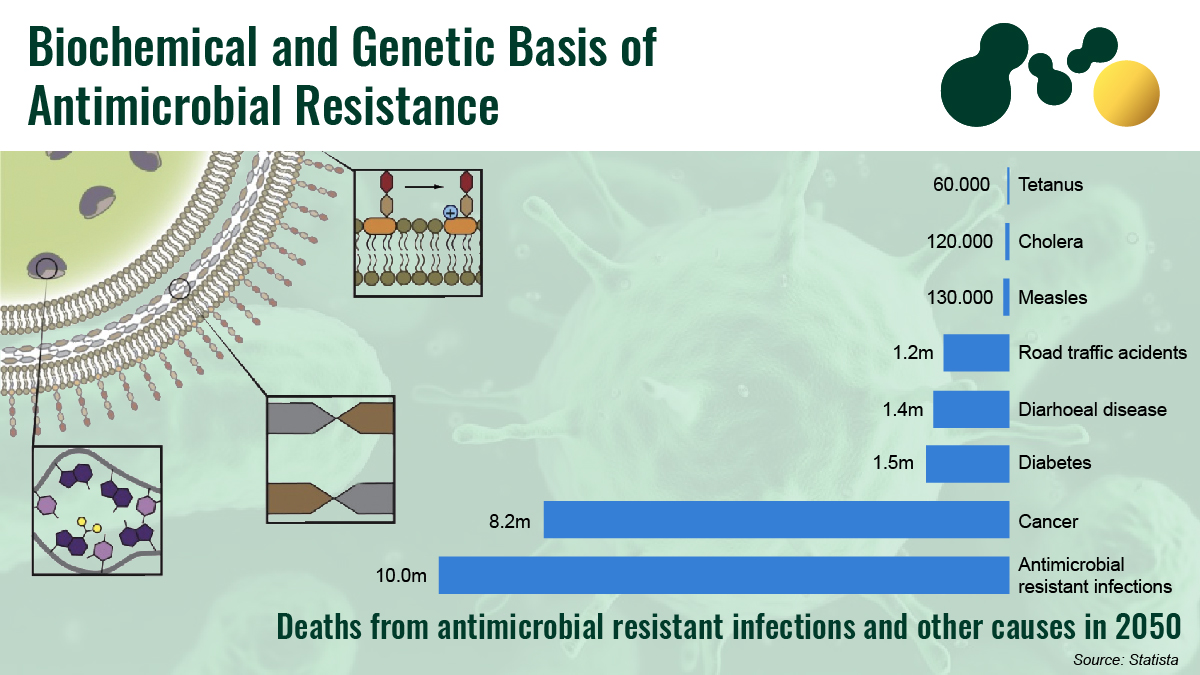
Morbidity and mortality are significant consequences of antimicrobial resistance in patients. Resistant organisms tend to multiply the chances of serious health issues and double the risks of death. Across the globe, the current number of people that lose their lives due to drug-resistant infections is approximately 700,000, with 2 million people affected in the United States each year and about 23,000 deaths occurring. This number is roughly similar to that of the European Union, with an annual mortality rate of 25,000.
Official reports estimate about 10 million deaths globally by 2050 if effective and strong actions are not taken against antimicrobial resistance (AMR). The cost of AMR is also extremely high worldwide, although it is different for each country. Recent researches indicate the possibility of antimicrobial resistance elevating the poverty rate and seriously affecting low-income countries. The gap between developing and developed countries tends to become more pronounced, leading to a substantial increase in inequity.
Mechanisms of Antimicrobial Resistance
Biochemical and genetic basis of resistance are the major mechanisms of AMR encountered in clinical practice and these mechanisms fall into four main categories: (1) inactivation of the antimicrobial molecule; (2) target modification; (3) active drug efflux; (4) drug uptake limitation. Table 1 summarizes the mechanisms of antimicrobial resistance against different antimicrobial agents.
- Inactivation of the Antimicrobial Molecule
Microorganisms now produce enzymes that can inactivate drug molecules by destroying the molecule or adding certain chemical moieties to render the antimicrobial unable to interact with its target site. Scientists have described various modifying enzymes that catalyze different biochemical reactions, such as (i) acetylation, (ii) phosphorylation, and (iii) adenylation. Regardless of the catalyzed reaction, the overall effect is related to steric hindrance, decreasing the drug’s avidity for its target site, with the overall effect being higher bacterial MIC. For example, β-lactam resistance works on the mechanism of destroying the β-lactam molecules by the action of β-lactamases. They are enzymes that destroy amide bonds of the β-lactam ring to render the antimicrobial ineffective. Studies have identified over 1000 β-lactamases, with several others likely to be reported as bacterial evolution continues.
- Target Modification
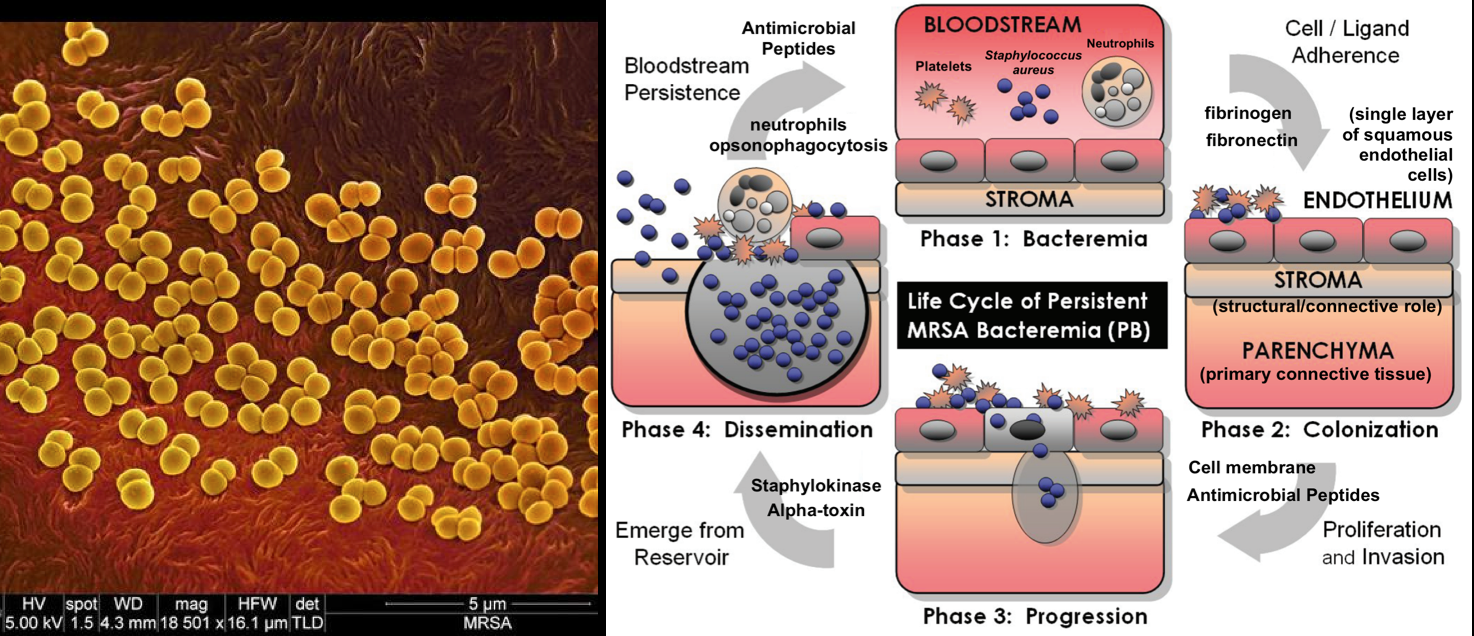
This is another major mechanism of resistance involving the modification of the antimicrobial target site to prevent the proper binding of the antimicrobial molecule. The target sites have crucial cellular functions in antimicrobial action. However, some mutational changes can occur on the target site to reduce inhibition susceptibility while retaining their cellular functions. In certain cases, modifying target structures to cause resistance will require other cellular changes to compensate for the alteration. A typical example is the acquisition of penicillin-binding transpeptidase (PBP2a) in methicillin-resistant Staphylococcus aureus.
- Active Drug Efflux
Some complex mechanisms can rapidly extrude toxic compounds out of the cell. Microorganisms can produce these machinery (efflux pumps) to result in antimicrobial resistance. These efflux pumps can affect every class of antibiotics because they all must be present intracellularly to exert their effects. Many efflux systems are capable of extruding a wide spectrum of unrelated molecules (multidrug transporters) to contribute immensely to multidrug resistance. There are different families of efflux pumps:
- major facilitator superfamily (MFS)
- small multidrug resistance family (SMR)
- resistance-nodulation cell division family (RND)
- ATP-binding cassette family (ABC)
- multidrug and toxic compound extrusion family (MATE)
- Drug Uptake Limitation
Bacteria have natural differences in their abilities to limit drug uptake. The composition of the outer membranes of some organisms (like gram-negative bacteria) slows down the penetration of antimicrobial agents and their transport across the water-filled channels of the membrane. Mycobacteria also have outer memberance with high lipid content making it difficult for hydrophilic drugs to penetrate them. Furthermore, organisms lacking cell wall (e.g., Mycoplasma) are intrinsically resistant to those agents that target cell walls. Formation of biofilms in pathogenic organisms will protect the microorganism for attacks by host’s immune system. Biofilms also provide adequate protection against antimicrobials agents.
| Drug | Drug Inactivation | Target Modification | Efflux Pumps | Drug Uptake Limitation |
| β-lactams | β-lactamases – Gram positive and Gram negative | Alterations in penicillin binding proteinss – Gram positive | RND | No outer cell wall, decreased porin number |
| Aminoglycosides | Acetylation, adenylation, phosphorylation, modifying enzymes | Methylation, ribosomal mutation | RND | Cell wall polarity |
| Tetracyclines | Oxidation, antibiotic modification | Ribosomal protection | RND, MFS | Decreased porin numbers |
| Macrolides | Methylation, ribosomal mutation | RND, ABC, MFS | ||
| Chloramphenicol | Drug acetylation | Ribosomal methylation | RND, MFS | |
| Glycopeptides | Modified peptidoglycan | No outer cell wall, thickened cell wall | ||
| Fluoroquinolones | Drug acetylation | Topoisomerase IV – Gram positive DNA gyrase modification – Gram negative | RND, MFS, MATE |
Table 1: A summary of antimicrobial resistance mechanisms against different classes of antimicrobial agents.
Origins of Antimicrobial Resistance
Microorganisms have remarkable genetic plasticity, allowing them to wade off varying environmental threats such as the presence of antimicrobial agents. Analysis of isolated microbial DNA from the dental plaque of human remains showed gene sequences similar to those conferring resistance to aminoglycosides, tetracycline, β-lactams, bacitracin, and macrolides in clinical strains (Warriner et al., 2014). Some organisms share similar ecology with antimicrobial-producing species, allowing them to develop mechanisms to withstand harmful antimicrobials.
- Mutational Resistance
In this case, the microbial cells of susceptible populations develop mutations in genes affecting drug activity, leading to cell survival, even when antimicrobial molecules are present.
- Spontaneous mutations
These are mutation events that occur randomly due to replication errors or incorrect repairs of damaged DNA strands in actively dividing cells. They are often referred to as growth-dependent mutations, and they present a vital mechanism for generating antimicrobial resistance. Spontaneous mutations are also nucleotide point mutations that occur at the same permissive growth time and can produce a resistance phenotype. For example, Escherichia coli has quinolone resistance due to changes in at least seven different gyrA gene positions but only three in the parC gene.
- Hypermutations
Some organisms now have elevated mutation rates (hyper mutators or hypermutable strains) among laboratory and natural populations. Studies have found that clinical and natural bacterial isolates now have a higher frequency of mutators than expected. This suggests that mutators may confer selective advantages in some situations in nature.
The most acceptable ‘hypermutable state’ model currently available states that some bacterial populations enter transient states of a high rate of mutation during prolonged non-lethal antibiotic pressure. Upon useful mutation to relieve the pressure, the cell grows and reproduces while remaining in the hypermutable state. While the trigger of hypermutation is still unclear, scientists believe that it is regulated by special inductively mutator DNA polymerases (pol) IV.
- Adaptive mutagenesis
Recent experimental data further shows that mutations can also occur in slowly dividing or non-dividing cells with some relation to the selective pressure introduced. These adaptive mutations only arise when there is non-lethal selective pressure present, favoring them. This feature differentiates them from spontaneous mutations and may be the major source of resistant mutants under natural conditions. From analysis, it is found that stress-enhanced mutagenesis is regulated. The major factors include stress-responsive, error-prone DNA polymerase IV (dinB) and V (umuCD), transiently increasing mutation rate.
- Horizontal Gene Transfer (HGT)
Another major driver of bacterial evolution is the acquisition of foreign materials through horizontal gene transfer. This mechanism is also responsible for developing antimicrobial resistance. Clinically relevant bacterial sharing a similar environment with natural antimicrobial agents have intrinsic genes for developing resistance. The simplest HGT type is transformation. However, some bacterial species can naturally incorporate naked DNA for the development of resistance.
Conjugation involves using mobile genetic elements (MGEs) to share genetic information, with the most important ones being transposing and plasmids. Another well-characterized mechanism is the direct chromosome-to-chromosome transfer. Finally, integrons represent some of the most efficient antimicrobial resistance mechanisms. They help to add newer genes to the bacterial chromosome with important machinery for expression.
Tackling Antimicrobial Resistance with Metagenomic Next Generation Sequencing (mNGS)
As routine microbiological screening includes resistant variants identification, the potential patient diagnosis waiting times should be as short as possible, especially for serious infections such as sepsis. However, in light of the overall AMR threat, not only the character of the resistance should be taken into account during pathogen ID in medical practice, but also the molecular mechanisms determining the occurrence and evolution of antimicrobial traits through genome sequencing.
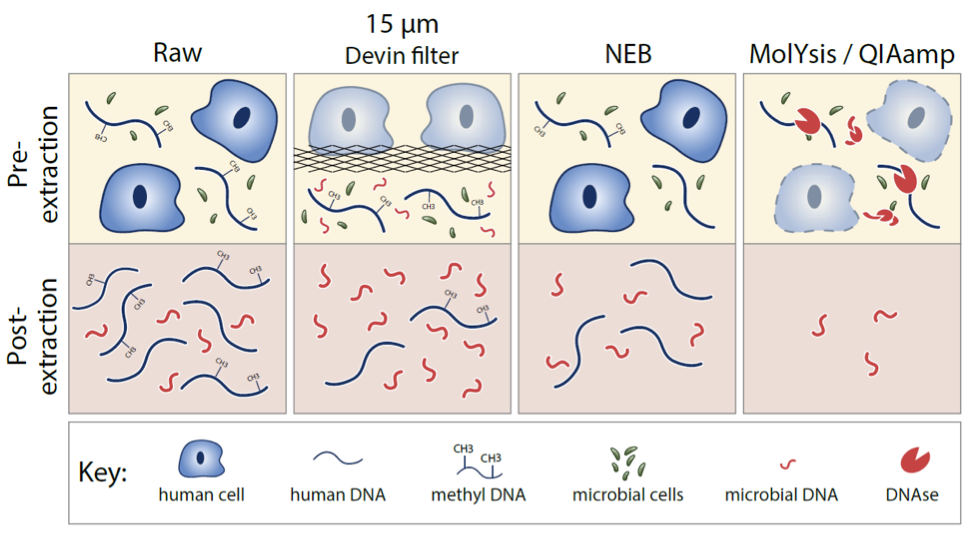
mNGS technologies (metagenomic Next Generation Sequencing) have become an essential tool for unbiased, culture-independent diagnosis as well as drug and diagnostic tests development by enabling the rapid identification and surveillance of resistance mechanisms. Including a complete genomic sequence provided by the metagenomics advancements represent the highest practicable level of structural detail on the individuating traits of an organism or population. It can be used to provide more reliable microbial identification, definitive phylogenetic relationships, and a comprehensive catalog of traits relevant for epidemiological investigations. This is having a major impact on outbreak investigations and the diagnosis and treatment of infectious diseases, as well as the practice of microbiology and epidemiology. However, the major bottleneck keeping mNGS from mass adoption in clinical microbiology, is human DNA contamination with patient’s genetic information depletion being the main cost in rapid pathogen ID and a timely treatment.
Devin® is a novel blood fractionation filter device that can be applied to efficiently deplete human cellular DNA background and enrich microorganisms in the whole blood samples in less than 5 minutes. With ten most frequent microorganisms causing bacteremia and fungemia in adults belonging to the Enterobacteriaceae, Staphylococcaceae and Streptococcaceae, families, whose AMR strains are listed by the CDC as either concerning, serious or urgent threats , the PaRTI-Seq® test, a metagenomic sequencing workflow built upon Devin® can identify these potential pathogens from whole blood samples within 24 hours with a sensitivity of 102 genome copies per milliliter. Increasing the scope of available genomic screening for the presence of antimicrobial resistance factors using PaRTI-Seq® and Devin® technology could not only speed up the diagnostic process, especially during BSI but also improve the efficiency of the AMR screening process, increasing existing antimicrobial databases with crucial data sharing and exchange. Devin® and PaRTI-Seq® have the potential for saving the sequencing cost significantly and constitute a faster method for AMR ID, especially from whole blood samples.
References
- D’Costa, V. M., McGrann, K. M., Hughes, D. W., and Wright, G. D. Sampling the antibiotic resistome. Science. 2006;311,374–377.
- Jim O’Neill. Tackling drug-resistant infections globally: final report and recommendations the review on antimicrobial resistance chaired. [Online] 2016. Available from: https://amr-review.org/sites/default/files/160518_Final paper_with cover.pdf.
- Founou RC, Founou LL, Essack SY. Clinical and economic impact of antibiotic resistance in developing countries: a systematic review and meta-analysis. PLoS One. [Online]. 2017;12:e0189621. Available from: https//doi.org/10.1371/journal.pone.0189621
- Shrestha P, Cooper BS, Coast J, et al. Enumerating the economic cost of antimicrobial resistance per antibiotic consumed to inform the evaluation of interventions affecting their use. Antimicrob Resist Infect Control. [Online]. 2018;7(1):98. Available from: doi:10.1186/s13756-018-0384-3
- World Bank. Drug-resistant infections a threat to our economic future. [Online] 2017. Available from: https://worldbank.org.
- Martinez JL. General principles of antibiotic resistance in bacteria. Drug Discov Today. 2014;11:33–39.
- Cox G, Wright GD. Intrinsic antibiotic resistance: mechanisms, origins, challenges and solutions. Int J Med Microbiol. 2013;303:287–292.
- Davies J. Where have all the antibiotics gone? Can J Infect Dis Med Microbiol. 2006;17:287–90.
- Wilson DN. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat Rev Microbiol. 2014;12:35–48.
- G. Spratt. Resistance to antibiotics mediated by target alterations. Science. 1994;264:388–393.
- Bush K. The ABCD’s of β-lactamase nomenclature. [Internet]. 2013;19:549–559. Available from: https://doi.org/10.1007/s10156-013-0640-7
- Bush K, Jacoby GA. Updated functional classification of β-lactamases. Antimicrob Agents Chemother. [Internet]. 2010;54:969–976. Available from: https://doi.org/10.1128/AAC.01009-09
- Warinner, C., Rodrigues, J. F., Vyas, R., Trachsel, C., Shved, N., Grossmann, J., et al. Pathogens and host immunity in the ancient human oral cavity. Nat. Genet. 2014;46:336–344.
- J. Piddock, R. Wise. Induction of the SOS response in Escherichia coli by 4-quinolone antimicrobial agents. FEMS Microbiol. Lett. 1987;41:289–294.
- Coculescu BI. Antimicrobial resistance induced by genetic changes. J Med Life. 2009;2:114–123.
- I. Aminov, R.I. Mackie. Evolution and ecology of antibiotic resistance genes. FEMS Microbiol. Lett. 2007;271:147– 161.
- Angers A., Petrillo M., Patak A., Querci M., Van den Eede G. The role and implementation of next-generation sequencing technologies in the coordinated action lan against antimicobial resistance. Joint Research Center. 2017;4-7.
- Schmidt, K., Mwaigwisya, S., Crossman, L.C., Doumith, M., Munroe, D., Pires, C., Khan, A.M., Woodford, N., Saunders, N.J., Wain, J., et al. Identification of bacterial pathogens and antimicrobial resistance directly from clinical urines by nanopore-based metagenomic sequencing. J. Antimicrob. Chemother. 2017;72:104–114.
- Köser et al. (2014). Whole-genome sequencing to control antimicrobial resistance. Trends Genet. 30(9):401–7.
- Han et al. (2019). mNGS in clinical microbiology laboratories: on the road to maturity. Crit Rev Microbiol 45(5-6):668-85.
- Balloux et al. (2018), From Theory to Practice: Translating WGS into the Clinic. Trends in Microbiology 26(2).
- Chen et al. (2021). Novel Human Cell Depletion Method For Rapid Pathogen ID by NGS. Labroots Microbiol. Week 10.13140/RG.2.2.23888.64007.
- CDC (2019). Antibiotic Resistance Threats in the US. Atlanta, GA: U.S. HHS Dept, cdc.gov/drugresistance/biggest-threats.html.