The validation of diagnostic assays is a long procedure of inter-related processes that assures all steps and reagents are optimised. The scope of this procedure is to detect the analyte with accuracy and precision and identify the thresholds for each parameter.
Three areas must be assessed: reproducibility (i.e., the test always shows the same result for the same sample), precision (i.e., the variability within results), and accuracy (i.e., the extent to which the results reflect the true situation). Once these are known, another three parameters need to be measured: scientific validity, analytical and clinical performance, guaranteeing data robustness and reliability.
The comparisons are done against a widely approved older technique, called golden standard.
A gold standard test is the best available diagnostic test or benchmark, under reasonable conditions.
Golden standards change, as technology progresses, increasing the quality of the standards and making the comparison ever so difficult to new kits to emerge.
The performance of a diagnostic test namely the sensitivity (or true positive rate TPR) and specificity. Diagnostic sensitivity and specificity are calculated in comparison to test results obtained from reference populations of known infection/exposure status. This mean that assay validation is a conditional process, i.e., it depends on how accurately the reference population is defined. The values of these two parameters are calculated as follows:

Related but not identical are the values of positive predictive value (PPV) and negative predictive value (NPV). Roughly, sensitivity and specificity evaluate the test, whereas the PPV and NPV evaluate the results. These two parameters are calculated as follows:
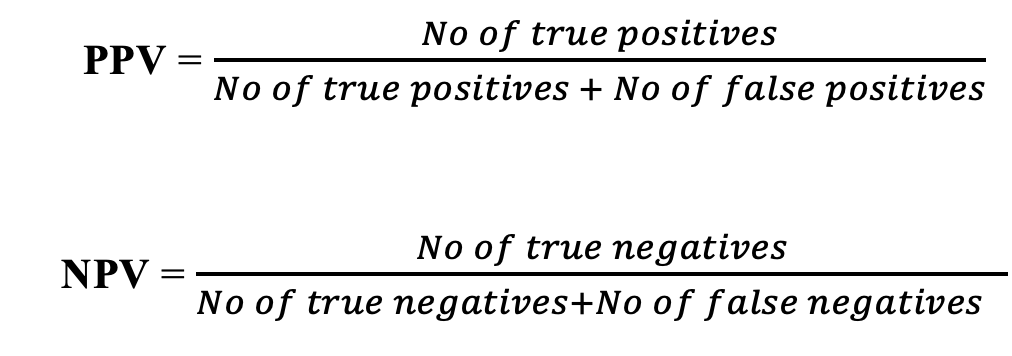
Another valuable parameter for microbiological tests is the Uncertainty of measurement (UM) which describes the range of uncertainty and can vary for the same test depending on the material tested, the collection, storage and shipment of the sample and the way of measurement (e.g., microplate reader).
Evaluation, Validation or Verification?
The aim of Evaluation is to compare different systems/test methods designed to perform the same or similar functions. Examples of microbiological evaluations include the comparison of different methods designed to detect the same marker/target, the comparison of different culture media to isolate the same organism, or the comparison of different equipment with the same function.
The scope of Validation, according to the ISO 15189:2012, is to ensure that the requirements for a specific intended use or application have been fulfilled, by providing objective evidence. Therefore, a Validation File must be produced which contains tests on known samples, workbooks, and relevant publications. A validation may be extensive or short depending on the validated item; a long validation is needed for a new in-house methodology, but a short is required for improvement of a already pre-existing commercial kit.
The ISO 15189:2012 also defines the Verification procedure as the confirmation of whether a product (for example commercial kit system or equipment) complies with a regulation, requirement, specification, or imposed condition. For example, verification should be performed when a laboratory wants to introduce for routine use a newly validated commercial method/equipment with defined performance (from manufacturer) or a modified and revalidated method.
In short, Validation is expected by the manufacturer of a commercial kit; Verification and Evaluation should be done by the User to ensure that it performs the way it is supposed to and suits the needs of the User, respectively.
Micronbrane Medical provides the solution with two products: Devin® and PaRTI-Seq® (Pathogen Real-Time Identification by Sequencing). Devin® membrane produces pathogen-enriched samples ready for downstream diagnostic applications. PaRTI-Seq® technology combines the latest third-generation sequencing (Nanopore Sequencing) and proprietary analytical methods, to provide test results within 22 hours.
References
- Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. The Lancet. 2021;397(10276):839-852. doi:10.1016/S0140-6736(21)00389-5
- Tomassetti C, D’Hooghe T. Endometriosis and infertility: Insights into the causal link and management strategies. Best Pract Res Clin Obstet Gynaecol. 2018;51:25-33. doi:10.1016/j.bpobgyn.2018.06.002
- Cousins FL, Pandoy R, Jin S, Gargett CE. The Elusive Endometrial Epithelial Stem/Progenitor Cells. Front Cell Dev Biol. 2021;9:640319. doi:10.3389/fcell.2021.640319
- Donnez J, Dolmans M-M. Endometriosis and Medical Therapy: From Progestogens to Progesterone Resistance to GnRH Antagonists: A Review. J Clin Med. 2021;10(5). doi:10.3390/jcm10051085
- Angioni S, D’Alterio MN, Coiana A, Anni F, Gessa S, Deiana D. Genetic Characterization of Endometriosis Patients: Review of the Literature and a Prospective Cohort Study on a Mediterranean Population. Int J Mol Sci. 2020;21(5). doi:10.3390/ijms21051765
- Hill CJ, Fakhreldin M, Maclean A, et al. Endometriosis and the Fallopian Tubes: Theories of Origin and Clinical Implications. J Clin Med. 2020;9(6). doi:10.3390/jcm9061905
- Agostinis C, Balduit A, Mangogna A, et al. Immunological Basis of the Endometriosis: The Complement System as a Potential Therapeutic Target. Front Immunol. 2021;11. doi:10.3389/fimmu.2020.599117, https://pubmed.ncbi.nlm.nih.gov/33505394/
- Laganà AS, Garzon S, Götte M, et al. The Pathogenesis of Endometriosis: Molecular and Cell Biology Insights. Int J Mol Sci. 2019;20(22). doi:10.3390/ijms20225615, https://pubmed.ncbi.nlm.nih.gov/31717614/
- Kobayashi H, Higashiura Y, Shigetomi H, Kajihara H. Pathogenesis of endometriosis: The role of initial infection and subsequent sterile inflammation (Review). Mol Med Rep. 2014;9(1):9-15. doi:10.38, https://pubmed.ncbi.nlm.nih.gov/24173432/92/mmr.2013.1755
- Koninckx PR, Ussia A, Tahlak M, et al. Infection as a potential cofactor in the genetic-epigenetic pathophysiology of endometriosis: a systematic review. Facts Views Vis ObGyn. 2019;11(3):209-216.
- Leonardi M, Hicks C, El-Assaad F, El-Omar E, Condous G. Endometriosis and the microbiome: a systematic review. BJOG Int J Obstet Gynaecol. 2020;127(2):239-249. doi:10.1111/1471-0528.15916