Invasive Candidiasis – a deadly disease
Candida spp., commensal yeasts that colonize in our gut and on our skin could become lethal pathogens when they overgrow and disseminate into our bloodstream and deep-seated organs (lung, liver, spleen, kidneys, bone, or eye) and then cause invasive candidiasis (IC). Bloodstream infection (BSI), also called candidemia and internal organ infection are two common forms of IC which are the predominant fungal hospital-acquired infections.
Candidemia is one of the most common hospital-associated infections with extremely high mortality rates among patients up to 75% worldwide
Additionally, the cost burden of the disease was substantial high. Invasive candidiasis was estimated as one of the highest healthcare costs of fungal diseases in the United States ($64,723–$153,090 per hospitalization).
Unfortunately, there estimated 3-5 cases with candidemia for every 100,000 persons in the general population, and even higher in hospital or ICU admissions.
Why is timely diagnosis important?
Long-term ICU stay, central venous catheter, parenteral nutrition, immunosuppression, broad spectrum antimicrobial treatment, recent major surgery, dialysis, diabetes are the most common risk factors associated with IC24. People developing IC usually have unknown fever, chill and do not respond successfully to most antibacterial drugs. These unspecific and unclear clinical signs and symptoms make diagnosis be challenging. Thus, the healthcare providers must rely on risk factors and symptoms and pathogen tests to determine IC.
Many studies showed that delayed diagnosis or misidentifications of Candida spp. led to delaying of initial treatment or inadequate treatment which were associated with the increase of death cases. It emphasizes that the rapid diagnostic methods are crucial to the improvement of outcomes of patients with Candida bloodstream infections.
The aetiological shift is also a reason for demanding of a diagnostic test with high specificity to detect the resistance and choose the optimal treatment regimens. Among various Candida pathogens, the five major causes of IC are Candida albicans, Candida glabrata, Candida tropicalis, Candida parapsilosis and Candida krusei . Whereas C. Albicans still remains the first rank in prevalence, there has been an epidemiological shift in non-albicans Candida species in the last decades. Different Candida spp. differ substantially in virulence and drug susceptibility. Although C. Albicans is susceptible to most of antifungal drugs, other Candida species pose considerable multidrug resistance. Moreover, a previously rare Candida pathogen, Candida auris, showed high resistance to fluconazole, amphotericin B and caspofungin, has globally spreading in more than 30 countries on six continents.
Current trends in diagnostic tests
There are some approaches applied worldwide to detect Candida spp., each one has its own advantages and drawbacks(Table 1).
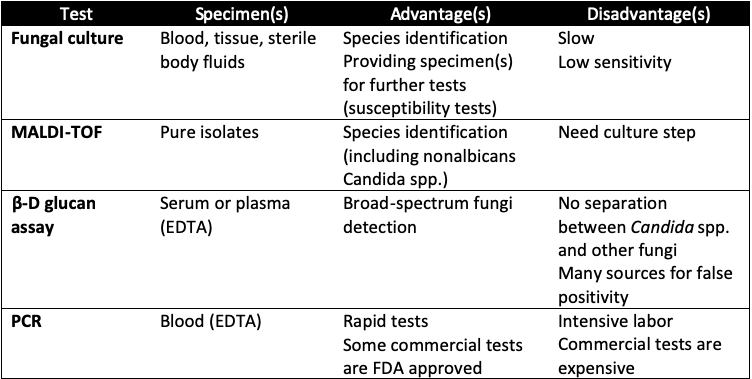
Table 1. Specimen types and invasive candidiasis diagnostic tests
According to US CDC, fungal culture from normally sterile sites such as blood, peritoneal fluid, and pleural fluid is widely used in clinicals as the gold standard test. However, the sensitivity of this method is only approximately 50% and the time to positivity ranges from 1 to 7 days. This is not the ideal candidate for timely diagnosis.
In recent years, three techniques approved by FDA may be helpful for IC diagnosis: matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF), the β-D glucan (Fungitell, Associates of Cape Cod, Inc, East Falmouth, MA) assay, and the T2Candida assay (T2 Biosystems, Lexington, MA).
MALDI-TOF uses mass spectroscopy to identify species in pure isolates. Its turnaround time for species identification is 10 – 15 minutes once the pure isolates are provided. It can determine non-albicans Candida spp. but still need culture step.
β-D glucan assay is a pan-fungal invasion test which cannot distinguish between Candida spp. and other fungi. Although the sensitivity of this assay ranged from 76.7% to 100%, the specificity varied from 40% to 91.8%. The limitations of this approach are non-specificity, high cost and difficulty in performance.
T2 Candida assay is magnetic resonance assay based on PCR technique which can directly detect five common candidemia pathogens (C. albicans, C. tropicalis, C. parapsilosis, C. krusei, and C. glabrata) in whole blood samples in as soon as 3 to 4 hours. However, the test results are categorized into three groups (not singe species determination): C. albicans or C. tropicalis; C. glabrata, C. krusei, S. cerevisiae or C. bracarensis; or C. parapsilosis, C. metapsilosis or C. orthopsilosis.
Other PCR-based assays are effective diagnostic tests with high sensitivity, single species identification and timeliness, compared with culture methods . Until now, the most thoroughly validated PCR-based system is SeptiFast (Roche, Basel, Switzerland). This multiplex real-time PCR test has been reported in some studies in Europe to detect 19 bacteria and 6 fungi (C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, C. krusei, and Aspergillus fumigatus) with sensitivity up to 94% . However, these assays still not widely validated in large-scale studies and cannot identify many Candida spp.
Nanopore sequencing is a promising method which can detect the missed or not detectable pathogens within 6 hours (including pre-sequencing preparation). It was also an expeditious tool to analyze the epidemiology of the C. auris outbreak.
With continuously improvement, Nanopore method will be the game changer in the effort of increasing the survival rates of patients with IC and minimizing economic burden as well.
Micronbrane Medical provides easy and rapid solutions with two products: Devin® and PaRTI-Seq® (Pathogen Real-Time Identification by Sequencing). Devin® membrane produces pathogen-enriched samples ready for downstream diagnostic applications. PaRTI-Seq® technology combines the latest third-generation sequencing (Nanopore Sequencing) and proprietary analytical methods, to provide test results within 22 hours.
References
- Benedict, K., Jackson, B. R., Chiller, T., & Beer, K. D. (2019). Estimation of Direct Healthcare Costs of Fungal Diseases in the United States. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 68(11), 1791-1797. doi:10.1093/cid/ciy776
- Chan, W. S., Au, C. H., Leung, S. M., Ho, D. N., Wong, E. Y. L., To, M. Y., . . . Tang, B. S. F. (2020). Potential utility of targeted Nanopore sequencing for improving etiologic diagnosis of bacterial and fungal respiratory infection. Diagnostic Pathology, 15(1), 41. doi:10.1186/s13000-020-00960-w
- Chen, J., Tian, S., Han, X., Chu, Y., Wang, Q., Zhou, B., & Shang, H. (2020). Is the superbug fungus really so scary? A systematic review and meta-analysis of global epidemiology and mortality of Candida auris. BMC infectious diseases, 20(1), 827-827. doi:10.1186/s12879-020-05543-0
- Cleveland, A. A., Harrison, L. H., Farley, M. M., Hollick, R., Stein, B., Chiller, T. M., . . . Park, B. J. (2015). Declining incidence of candidemia and the shifting epidemiology of Candida resistance in two US metropolitan areas, 2008-2013: results from population-based surveillance. PLoS ONE, 10(3), e0120452. doi:10.1371/journal.pone.0120452
- Cohen, J. F., Ouziel, A., Matczak, S., Brice, J., Spijker, R., Lortholary, O., . . . Toubiana, J. (2020). Diagnostic accuracy of serum (1,3)-beta-d-glucan for neonatal invasive candidiasis: systematic review and meta-analysis. Clinical Microbiology and Infection, 26(3), 291-298. doi:https://doi.org/10.1016/j.cmi.2019.09.010
- Doğan, Ö., Yeşilkaya, A., Menekşe, Ş., Güler, Ö., Karakoç, Ç., Çınar, G., . . . Ergönül, Ö. (2020). Effect of initial antifungal therapy on mortality among patients with bloodstream infections with different Candida species and resistance to antifungal agents: A multicentre observational study by the Turkish Fungal Infections Study Group. Int J Antimicrob Agents, 56(1), 105992. doi:10.1016/j.ijantimicag.2020.105992
- Ellepola, A. N., & Morrison, C. J. (2005). Laboratory diagnosis of invasive candidiasis. J Microbiol, 43 Spec No, 65-84.
- Garey, K. W., Rege, M., Pai, M. P., Mingo, D. E., Suda, K. J., Turpin, R. S., & Bearden, D. T. (2006). Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 43(1), 25-31. doi:10.1086/504810
- Kaur, H., & Chakrabarti, A. (2017). Strategies to Reduce Mortality in Adult and Neonatal Candidemia in Developing Countries. Journal of Fungi, 3. doi:10.3390/jof3030041
- Koehler, P., Stecher, M., Cornely, O. A., Koehler, D., Vehreschild, M., Bohlius, J., . . . Vehreschild, J. J. (2019). Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin Microbiol Infect, 25(10), 1200-1212. doi:10.1016/j.cmi.2019.04.024
- Korber, F., Zeller, I., Grünstäudl, M., Willinger, B., Apfalter, P., Hirschl, A. M., & Makristathis, A. (2017). SeptiFast versus blood culture in clinical routine – A report on 3 years experience. Wiener klinische Wochenschrift, 129(11-12), 427-434. doi:10.1007/s00508-017-1181-3
- Kullberg, B. J., & Arendrup, M. C. (2015). Invasive Candidiasis. New England Journal of Medicine, 373(15), 1445-1456. doi:10.1056/NEJMra1315399
- Labelle, A. J., Micek, S. T., Roubinian, N., & Kollef, M. H. (2008). Treatment-related risk factors for hospital mortality in Candida bloodstream infections*. Critical Care Medicine, 36(11), 2967-2972. doi:10.1097/CCM.0b013e31818b3477
- Lacroix, C., Gicquel, A., Sendid, B., Meyer, J., Accoceberry, I., François, N., . . . Bougnoux, M. E. (2014). Evaluation of two matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) systems for the identification of Candida species. Clinical Microbiology and Infection, 20(2), 153-158. doi:https://doi.org/10.1111/1469-0691.12210
- Lamoth, F., Lockhart, S. R., Berkow, E. L., & Calandra, T. (2018). Changes in the epidemiological landscape of invasive candidiasis. J Antimicrob Chemother, 73(suppl_1), i4-i13. doi:10.1093/jac/dkx444
- McCarty, T. P., & Pappas, P. G. (2016). Invasive Candidiasis. Infectious Disease Clinics, 30(1), 103-124. doi:10.1016/j.idc.2015.10.013
- McMullan, R., Metwally, L., Coyle, P. V., Hedderwick, S., McCloskey, B., O’Neill, H. J., . . . Hay, R. J. (2008). A Prospective Clinical Trial of a Real-Time Polymerase Chain Reaction Assay for the Diagnosis of Candidemia in Nonneutropenic, Critically Ill Adults. Clinical Infectious Diseases, 46(6), 890-896. doi:10.1086/528690
- Morrell, M., Fraser, V. J., & Kollef, M. H. (2005). Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother, 49(9), 3640-3645. doi:10.1128/aac.49.9.3640-3645.2005
- Muderris, T., Kaya, S., Ormen, B., Aksoy Gokmen, A., Varer Akpinar, C., & Yurtsever Gul, S. (2020). Mortality and risk factor analysis for Candida blood stream infection: A three-year retrospective study. Journal de Mycologie Médicale, 30(3), 101008. doi:https://doi.org/10.1016/j.mycmed.2020.101008
- Mylonakis, E., Clancy, C. J., Ostrosky-Zeichner, L., Garey, K. W., Alangaden, G. J., Vazquez, J. A., . . . Pappas, P. G. (2015). T2 Magnetic Resonance Assay for the Rapid Diagnosis of Candidemia in Whole Blood: A Clinical Trial. Clinical Infectious Diseases, 60(6), 892-899. doi:10.1093/cid/ciu959
- Nguyen, M. H., Wissel, M. C., Shields, R. K., Salomoni, M. A., Hao, B., Press, E. G., . . . Clancy, C. J. (2012). Performance of Candida real-time polymerase chain reaction, β-D-glucan assay, and blood cultures in the diagnosis of invasive candidiasis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 54(9), 1240-1248. doi:10.1093/cid/cis200
- Osman, M., Al Bikai, A., Rafei, R., Mallat, H., Dabboussi, F., & Hamze, M. (2020). Species distribution and antifungal susceptibility patterns of clinical Candida isolates in North Lebanon: A pilot cross-sectional multicentric study. J Mycol Med, 30(3), 100986. doi:10.1016/j.mycmed.2020.100986
- Pappas, P. G., Kauffman, C. A., Andes, D. R., Clancy, C. J., Marr, K. A., Ostrosky-Zeichner, L., . . . Sobel, J. D. (2015). Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clinical Infectious Diseases, 62(4), e1-e50. doi:10.1093/cid/civ933
- Pappas, P. G., Lionakis, M. S., Arendrup, M. C., Ostrosky-Zeichner, L., & Kullberg, B. J. (2018). Invasive candidiasis. Nature Reviews Disease Primers, 4(1), 18026. doi:10.1038/nrdp.2018.26
- Quindós, G. (2014). Epidemiology of candidaemia and invasive candidiasis. A changing face. Rev Iberoam Micol, 31(1), 42-48. doi:10.1016/j.riam.2013.10.001
- Rhodes, J., Abdolrasouli, A., Farrer, R. A., Cuomo, C. A., Aanensen, D. M., Armstrong-James, D., . . . Schelenz, S. (2017). Rapid genome sequencing for outbreak analysis of the emerging human fungal pathogen <em>Candida auris</em>. bioRxiv, 201343. doi:10.1101/201343